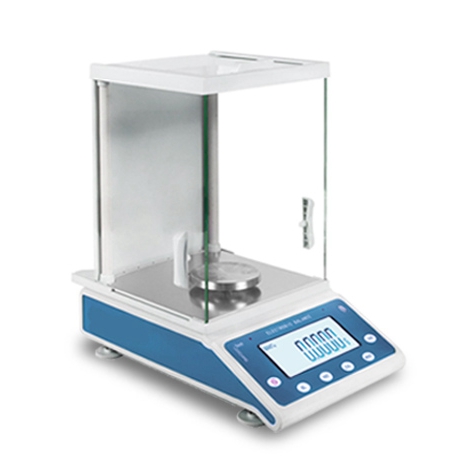
The analytical balance, also known as a precision balance, is a crucial instrument in laboratory settings, particularly in fields requiring meticulous measurement such as chemistry, biology, and material science. Its primary use is for quantitative chemical analysis due to its high degree of accuracy, typically ranging from 0.1 to 0.01 milligrams. Below are detailed explanations regarding the uses of an analytical balance in a laboratory:
1. Fundamental Purpose
The fundamental purpose of an analytical balance is to measure the mass of substances with extreme precision. This precision is vital for experiments where small mass variations can significantly impact the results.
2. Applications in Chemical Analysis
- Quantitative Analysis: In chemical analysis, the analytical balance is used to determine the exact mass of reactants, products, or samples. This is crucial for stoichiometric calculations, ensuring the accuracy of reaction yields and product purity.
- Preparation of Solutions: When preparing solutions, the analytical balance helps in measuring the solute mass precisely, which is essential for achieving the desired concentration.
- Material Characterization: In material science, the balance is used to measure the mass of materials before and after treatments, aiding in the understanding of material properties and transformations.
3. Types and Operation
Analytical balances are available in various types, including mechanical and electronic models. Electronic analytical balances often incorporate advanced features such as automatic calibration, temperature compensation, and data recording.
- Mechanical Analytical Balance: Operates based on the lever principle. It requires manual calibration and may be less accurate than electronic models but is still reliable for certain applications.
- Electronic Analytical Balance: Utilizes electromagnetic force to balance the load and is highly automated. It often includes features like auto-calibration, data logging, and connectivity options for integration with laboratory information management systems (LIMS).
4. Importance of Precision
The precision of an analytical balance is paramount as it directly affects the reliability and reproducibility of experimental results. Even minute errors in measurement can lead to significant deviations in experimental outcomes, making it essential to regularly verify and maintain the accuracy of the balance.
In conclusion, the analytical balance is an indispensable tool in laboratory settings, enabling scientists and researchers to perform precise measurements that are critical for accurate chemical analysis, material characterization, and various other experimental procedures.
 Top Lab Equip
Top Lab Equip
